Рецевмо / Retsevmo / селперкатініб / Selpercatinib 40 мг 120 таб. Генеріг, Бангладешь
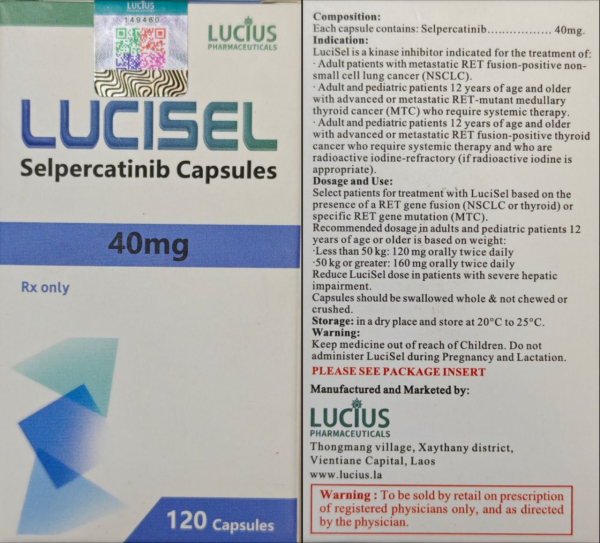
Indication
Selpercatinib is approved to treat:
adult with locally advanced or metastatic non-small cell lung cancer (NSCLC) with a rearranged during transfection (RET) gene fusion.9 In Europe, patients should require systemic therapy following prior treatment with immunotherapy and/or platinum-based chemotherapy.8
adults and children 2 years of age and older with advanced or metastatic medullary thyroid cancer (MTC) with a RET mutation who require systemic therapy.9 In Europe, patients should be ≥12 years of age and previously treated with sorafenib, lenvatinib, cabozantinib and/or vandetanib.8
adults and children 2 years of age and older with advanced or metastatic thyroid cancer with a RET gene fusion who require systemic therapy and who are radioactive iodine-refractory (if radioactive iodine is appropriate) 9
adults and children 2 years of age and older with locally advanced or metastatic solid tumors with a RET gene fusion that have progressed on or following prior systemic treatment or who have no satisfactory alternative treatment options 9
Selpercatinib is currently approved for these indications under an accelerated approval scheme and continued approval may be contingent on future confirmatory trials.7
-
Commercial name:Рецевмо
-
Сhemical name:селперкатініб
-
Dosage:40 mg
-
Quantity:120 таб
-
Release form:Таб
-
Производитель:Lucius, Бангладеш